
RENOVA-RDX
Digital organization between R&D, Regulatory Affairs and associated departments. Maximize operational efficiency while assuring compliance with both internal corporate and external regulatory standards.
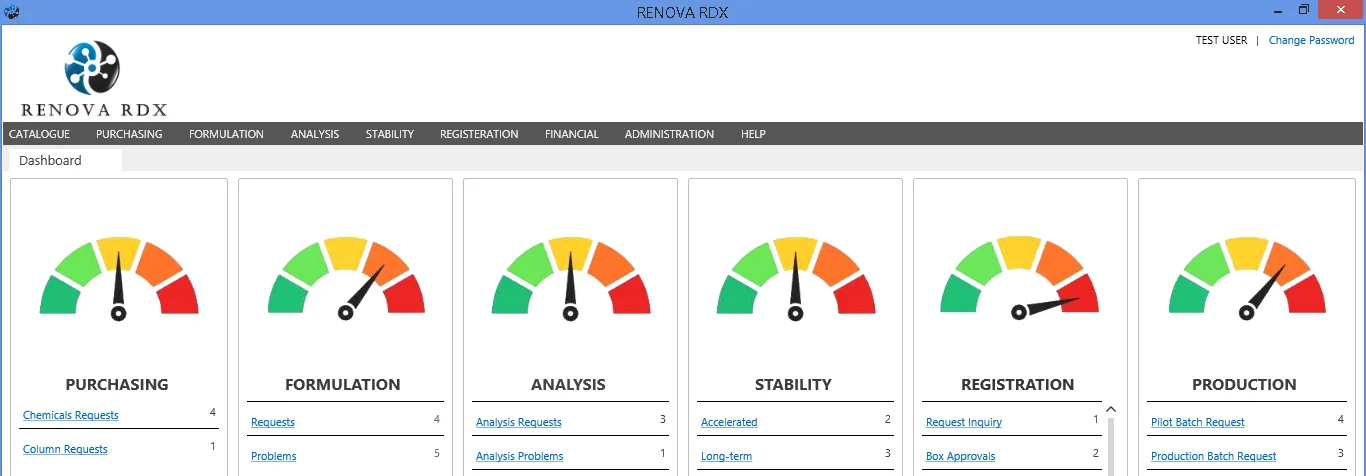
What is RENOVA-RDX?
RENOVA RDX is a modern integrated platform designed to bridge the gap between Pharmaceutical R&D and Regulatory Affairs.
It helps manufacturers maximize operational efficiency while maintaining strict compliance. With task management capabilities, it grants users throughout the facility appropriate access to input and retrieve data from a single, centralized system. Whether you are managing formulation, stability studies, or regulatory submissions, RENOVA-RDX simplifies the entire lifecycle.
Integrated Modules
Comprehensive solutions for every stage of product development.
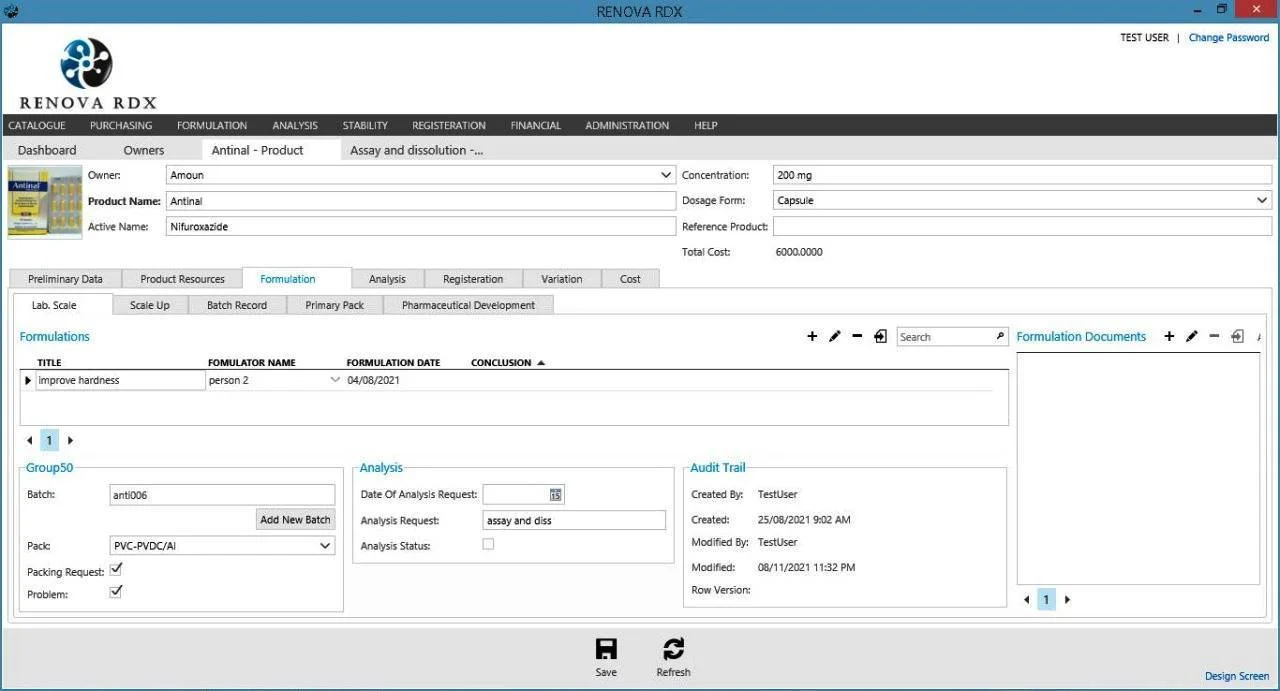
Formulation Module
Manage product development from concept to pilot. Track active ingredients, excipients, and process parameters.
- Recipe management and version control
- Bill of Materials (BOM) optimization
- Process parameter tracking
- Seamless scale-up to manufacturing
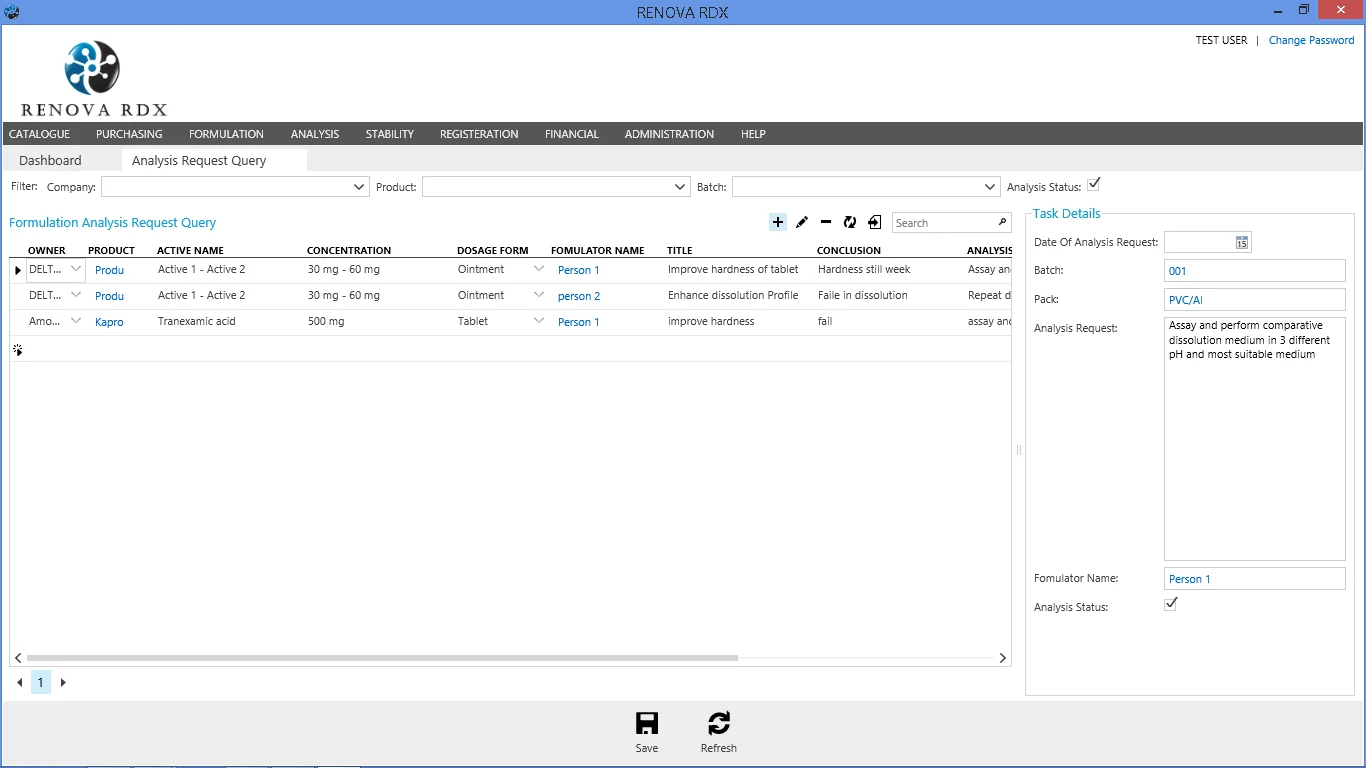
Analysis Module
Comprehensive analytical data management for raw materials, intermediates, and finished products.
- Method validation management
- Specification tracking
- Certificate of Analysis (CoA) generation
- Integration with laboratory instruments
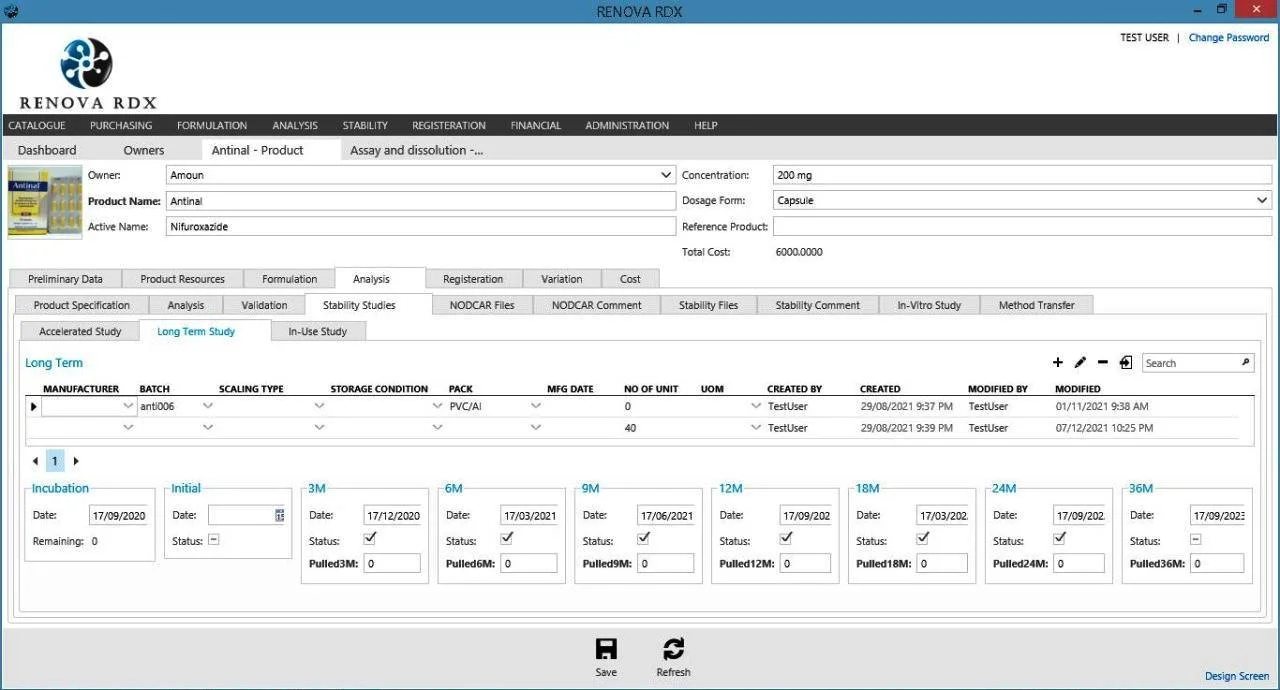
Stability Module
Manage stability studies with precision. Schedule testing intervals, track samples, and analyze degradation trends.
- Automated study scheduling & alerts
- Chamber management & monitoring
- Trend analysis & shelf-life prediction
- Compliant with ICH guidelines
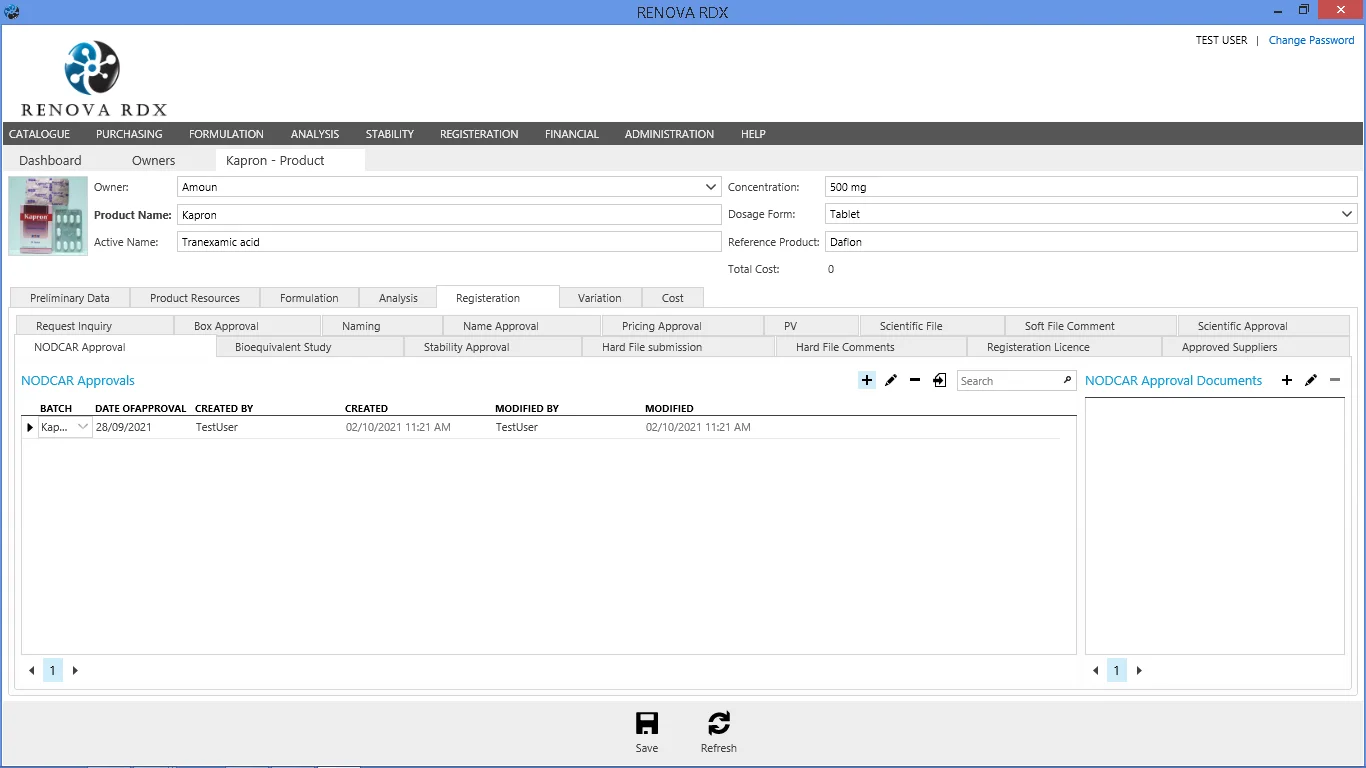
Regulatory Affairs Module
Streamline submission processes and ensure compliance with local and international regulations.
- Dossier management & compilation
- Registration tracking & renewal alerts
- Correspondence tracking with authorities
- eCTD support readiness
Accelerate Your R&D Pipeline
Contact our experts to see how RENOVA-RDX can streamline your development and regulatory processes.
Request a Demo