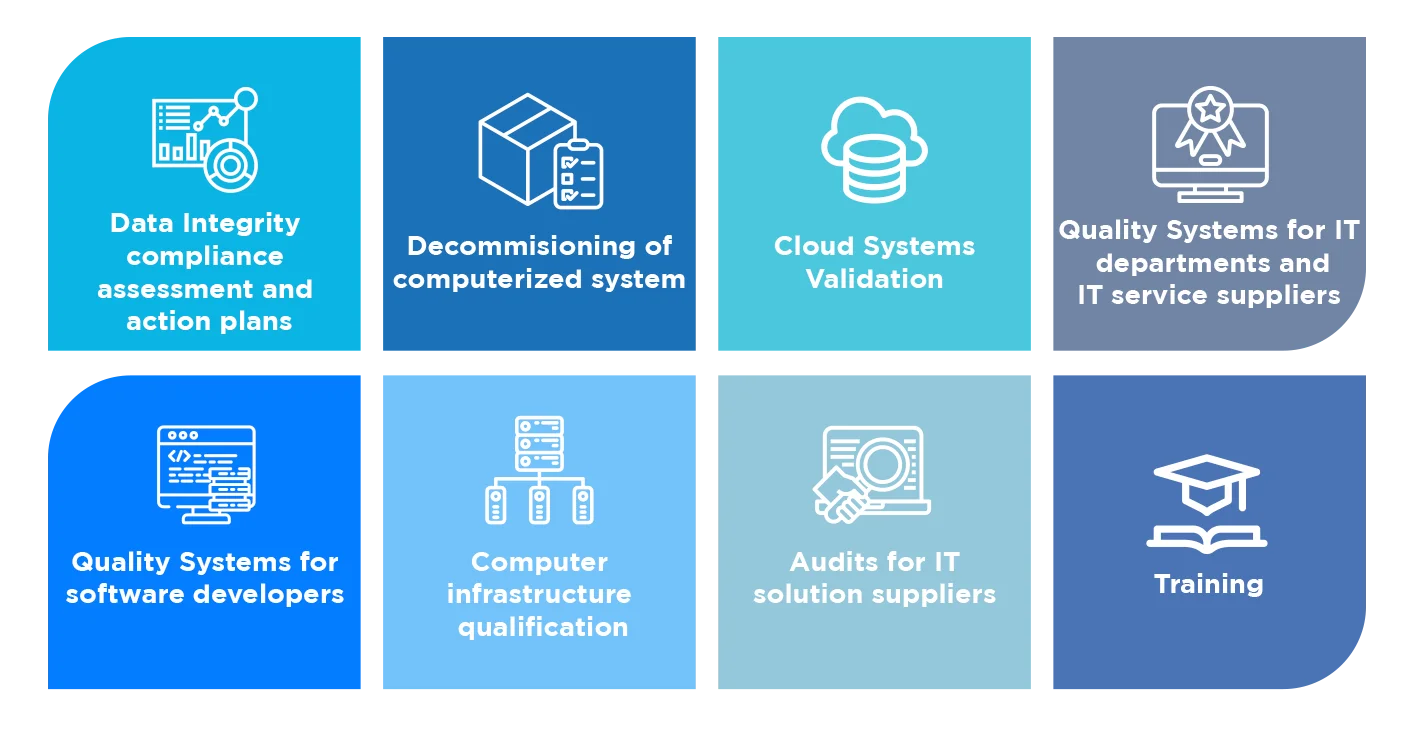
Computer System Validation (CSV)
Proving your systems perform as intended. Comprehensive validation services for GxP compliance and data integrity.
What Is Computer System Validation?
Software validation ensures the system fits its intended use. For laboratory informatics (LIMS, ELN, CDS) in regulated industries (pharma, food & beverage), validation is essential for product safety and data security. The FDA and other global agencies regulate industries that directly impact consumer health, mandating validation to ensure accuracy and reliability.










FDA CFR Part 11
In the US, 21 CFR Part 11 deals with electronic records and signatures. It mandates that electronic records/signatures must be accurate, reliable, readily retrievable, and secure to replace paper records legally. Validating your computer system is the primary means of proving compliance with these regulations.
The Validation Process
Validation spans the system life cycle. For new systems, it starts from the ground up. for existing systems or upgrades, it ensures the system remains in a validated state. The process ends only when the system is retired and data migrated/archived. It supports the entire project lifecycle.









The Validation Master Plan
The Validation Master Plan (VMP) guides the entire process. It includes requirements gathering, functional risk assessment, trace matrix, IQ/OQ/PQ protocols, and change control procedures. Each step is executed in order (Requirements -> Risk Assessment -> Trace Matrix -> Testing) to minimize risk and rework.
IQ/OQ/PQ Testing
Successful completion of Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ) verifies that the system functions as intended. Testing should be performed by qualified personnel familiar with lab informatics systems (LIMS, ELN, CDS) to ensure comprehensive coverage and compliance.

Ensure Your Compliance Today
Contact our experts for a comprehensive validation plan tailored to your regulatory needs.
Contact Us