MicroLIMS™
Microbiology Laboratory Information Management System.
Microbiology Laboratory Management
MicroLIMS is a sophisticated software solution designed to streamline and enhance the management of information within microbiology laboratories. Tailored to meet the unique needs of microbiologists, it offers features for sample tracking, data analysis, and result reporting.
MicroLIMS automates and centralizes the management of laboratory processes, from sample accessioning to quality control. It empowers researchers to focus on improved workflow, data integrity, and overall laboratory productivity.
Effortlessly Manage All Lab Information
The MicroLIMS portfolio empowers laboratories to do what they do best – make a difference. By offering standardized processes, optimized workflows, impactful analytics, and streamlined inventory management, laboratories can elevate their performance and improve patient care.
Comprehensive Microbiology Suite
MicroLIMS is an innovative solution covering microbiological examinations like test suitability, growth promotion tests (GPT), Microbiological Limits tests (MLT), Antibiotic bioassay, Sterility tests, Bacterial endotoxins, and preservative efficacy tests.
Common laboratory activities like media preparations, reference strains, and stock control are included, along with assets and instrument logs. Control of samples that are out of specifications (OOS) is fully managed.
Key Features
Designed for the specific needs of microbiology labs.
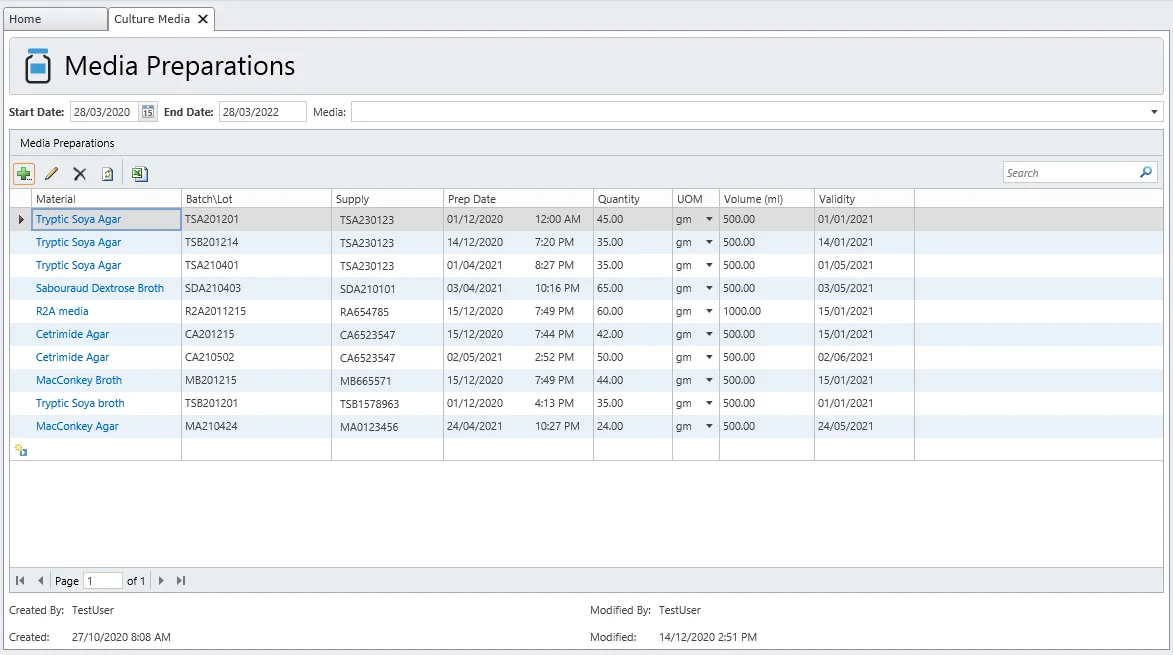
Media Preparations
Microbial culture media preparation is a routine task. MicroLIMS provides recording of all pertaining information for culture media, subculture of Certified Reference Microorganisms (CRMs), and other preparations that support integration of laboratory workflows.
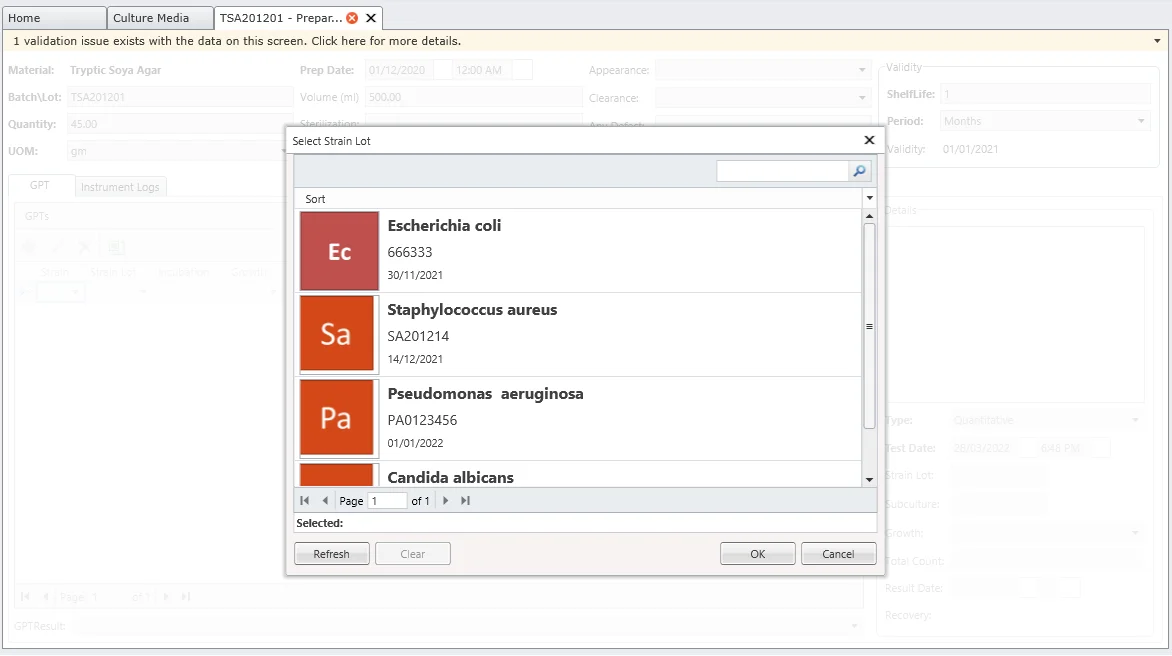
Growth Promotion Tests (GPT)
GPT confirms the ability of a new batch of media to support growth. MicroLIMS links media preparation data with associated GPTs to support metrological traceability for all media used in cGMP facilities.
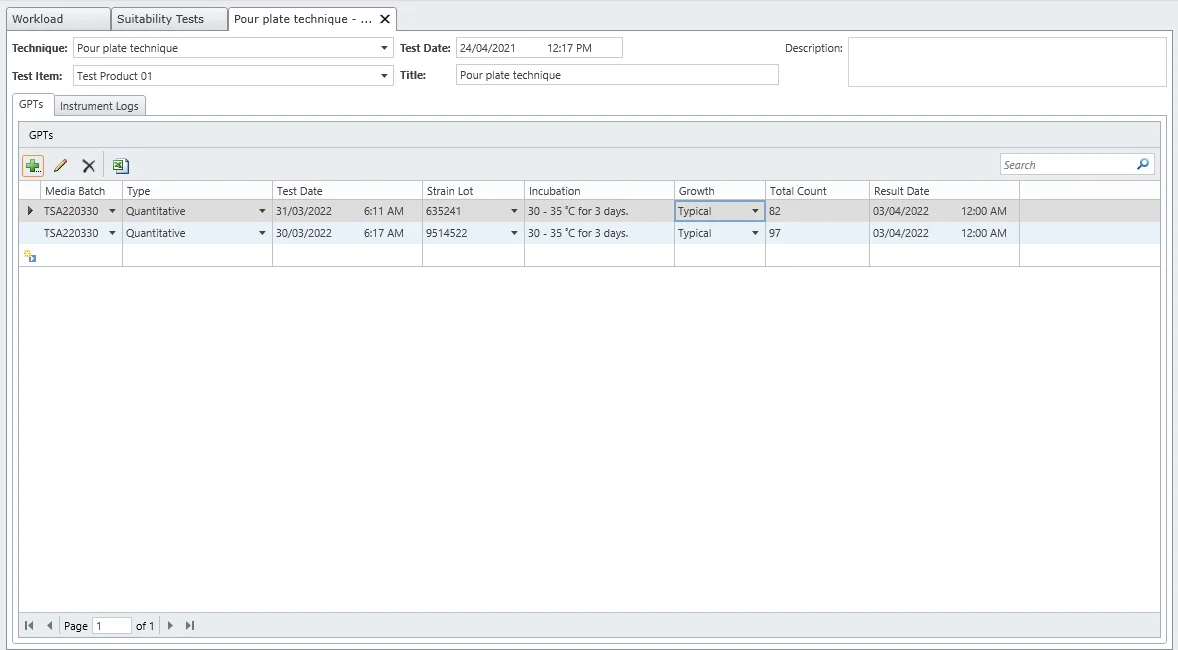
Suitability Tests
Demonstrate that any residual antimicrobial properties have been neutralized. MicroLIMS integrates pertaining information of varies suitability protocols, supporting direct exporting data and generation of printed documents (USP 41 compliant).
Microbial Limits Tests (MLT)
Assess viable microorganisms in non-sterile products. MicroLIMS integrates total aerobic microbial count (TAMC), total yeast and mold count, and tests for pathogenic microorganisms to determine compliance with established specifications.
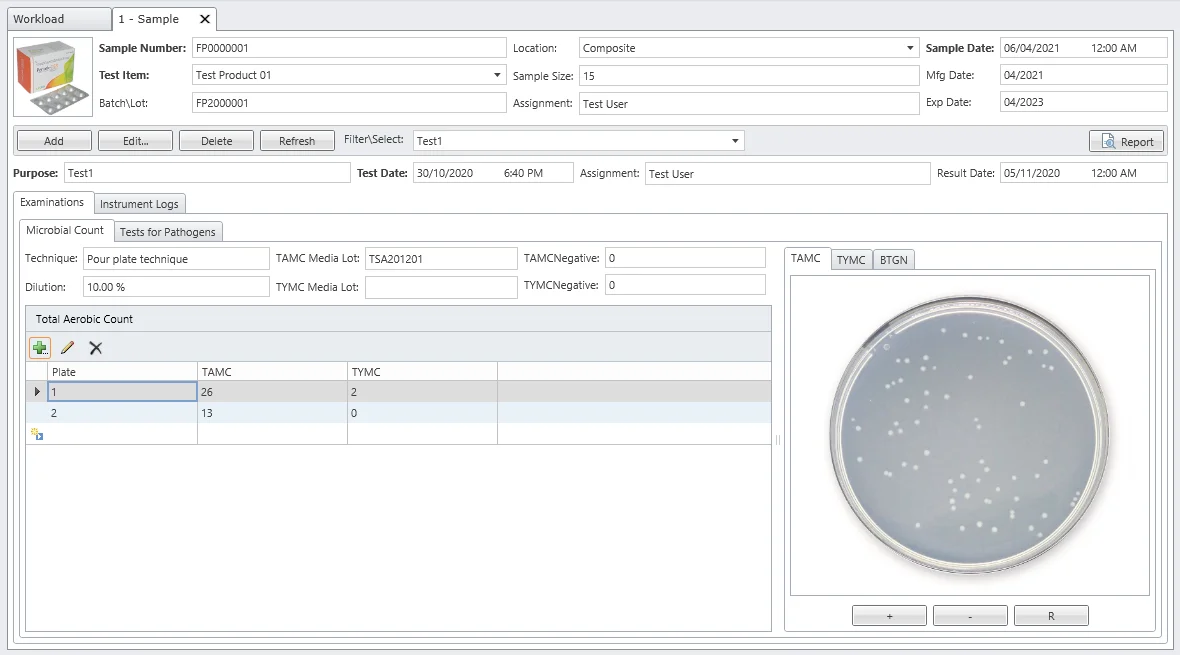
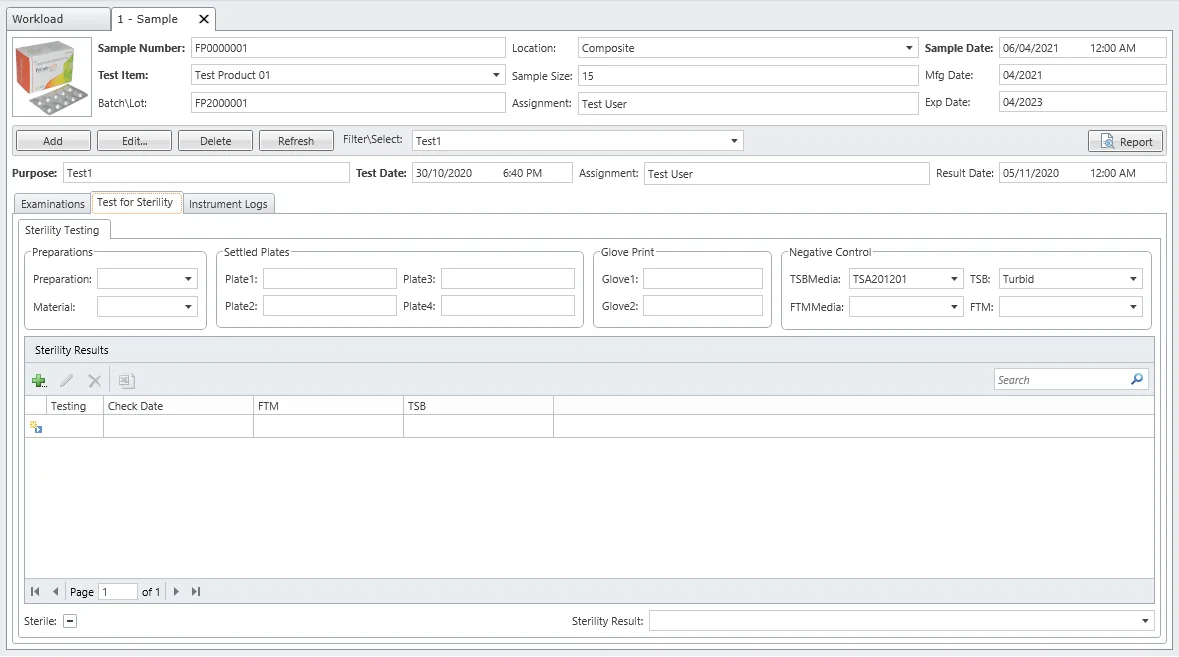
Sterility Tests
Confirm sterile products do not contain viable microorganisms before release. MicroLIMS supports accurate sterility testing methods (membrane filtration or direct inoculation) crucial for medical devices and pharmaceutical products.
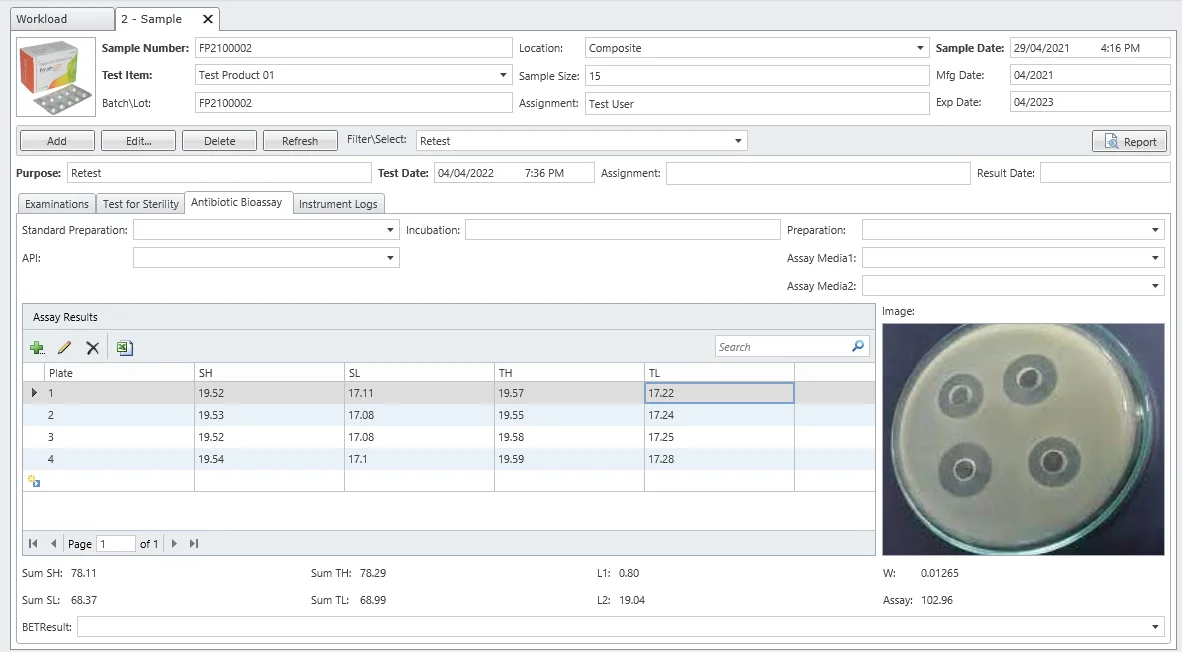
Antibiotic Bioassay
Estimate active constituents and biological activity of antibiotics. Microbiological assay helps in monitoring stability and resolving doubts regarding possible changes in potency that chemical methods may not detect.
Ready to Modernize Your Microbiology Lab?
Contact us today to discuss how MicroLIMS can support your specific needs.
Contact Us